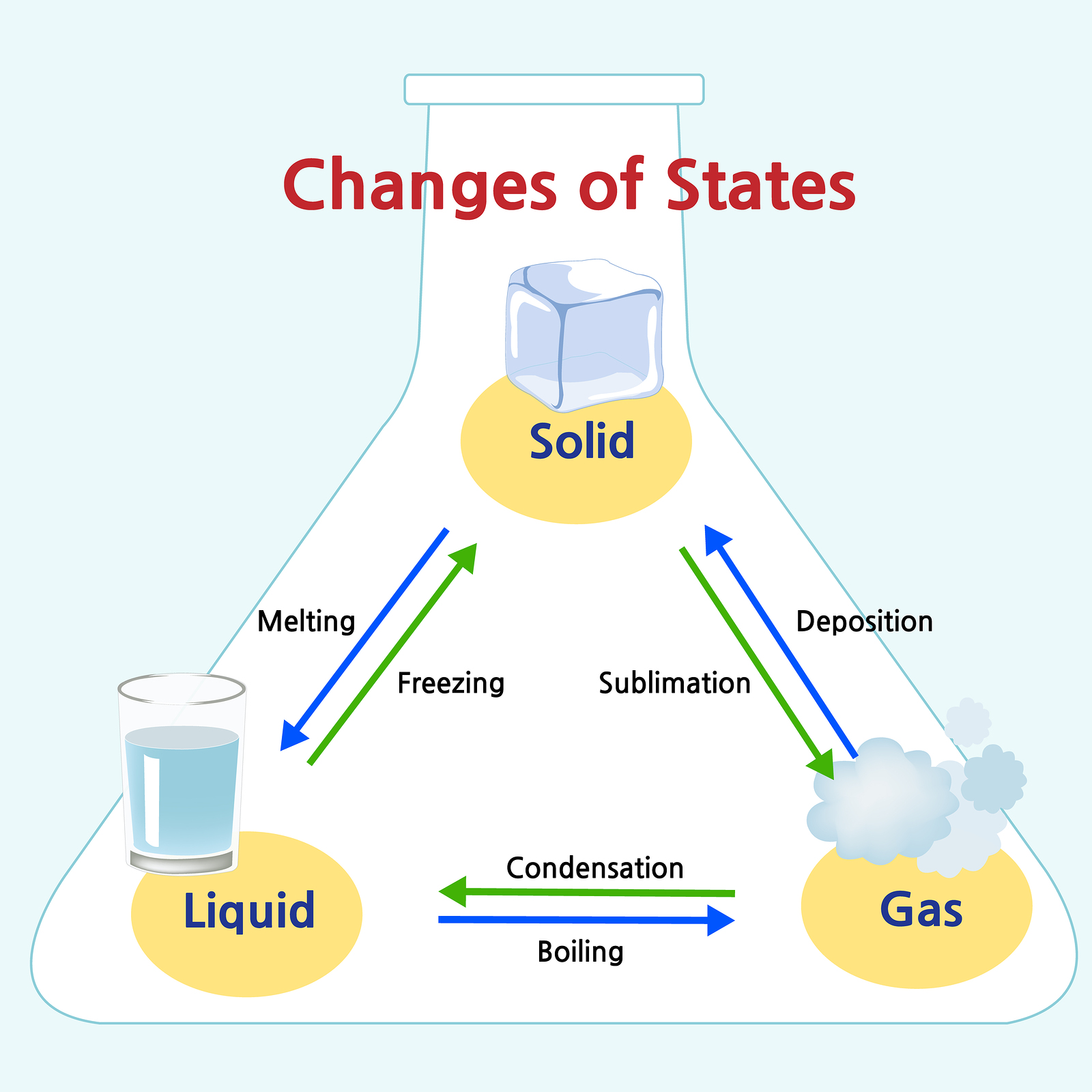
Solid Liquid Gas Chart
Solid Liquid Gas Chart - Matter is usually classified into three classical states. Solids, liquids, gases, and plasma. Highly strong intermolecular forces between the molecules, leads to a definite volume in solids. Web this is another great question. Web examples of liquids include water, juice, and vegetable oil. Like a liquid, a gas takes the shape of a container. Web solids and liquids have a fair bit in common, as in both states the molecules are joined together. Add or remove heat and watch the phase change. Web a vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid (or solid). Unlike a liquid, a gas easily expands or contracts to fill the entire volume of the container. Thus, there is no definite volume. Where solids and liquids differ is in their shape. Expand a little when heated. Connect daily observations to molecular interactions using electronegativity, bond polarity, and intermolecular forces. Web difference between solid liquid and gases; The intermolecular forces are stronger than gases but weaker than solids. Web we recommend using the latest version of chrome, firefox, safari, or edge. Web the atoms and molecules in gases are much more spread out than in solids or liquids. Liquid is a substance, that flows freely, having a definite volume but no permanent shape. Liquid vibrate, move about, and slide past each other. Gases have far less in common, on a molecular level, than solids and liquids. Liquid is a substance, that flows freely, having a definite volume but no permanent shape. Liquid are close together with no regular arrangement. They can be crystalline, like table salt, or amorphous, like glass, rubber or plastic. Web gas are well separated with no regular arrangement. Particles in a gas have more energy than in solids or. Web reimagine the everyday with a closer look at the states of matter! A gas is a state of matter lacking either a defined volume or defined shape. Liquid are close together with no regular arrangement. Density very low density of a substance in usually lower in the liquid. Web they don’t pour like a liquid. Solids are incompressible and have high density, compared to liquids and gases. Liquid vibrate, move about, and slide past each other. They can be crystalline, like table salt, or amorphous, like glass, rubber or plastic. Gas vibrate and move freely at high speeds. No expand when heated expand greatly when heated. Web heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In this state, the distinction between liquid and gas disappears. Solids are incompressible and have high density, compared to liquids and gases. Thus, there is no definite volume. Web compare three states of matter: Gas vibrate and move freely at high speeds. Some substances exist as gases at room. For example, the addition of heat can melt ice into liquid water and turn that water into. Web heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Web solids and liquids have a fair bit in common, as in both states the molecules are joined together. Web aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Relate the interaction potential to the forces. Web the atoms. Gases have far less in common, on a molecular level, than solids and liquids. Web the atoms and molecules in gases are much more spread out than in solids or liquids. As such, they can both be weighed, and have a fixed volume. Direct the children of grade 2 and grade 3 to observe the illustrations given in this circle.. The state that a given substance exhibits is also a physical property. Web they don’t pour like a liquid. Web matter typically exists in one of three states: Highly strong intermolecular forces between the molecules, leads to a definite volume in solids. Web solids and liquids have a fair bit in common, as in both states the molecules are joined. No expand when heated expand greatly when heated. Web yuji kotani / getty images. In ancient greece, one philosopher recognized how water could change form and reasoned that everything must be made of water. Web a vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid (or. Some substances exist as gases at room. Solids are incompressible and have high density, compared to liquids and gases. Web they don’t pour like a liquid. Web aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Matter is usually. Density very low density of a substance in usually lower in the liquid than in the solid state. Where solids and liquids differ is in their shape. Relate the interaction potential to the forces. Expand a little when heated. Gases have far less in common, on a molecular level, than solids and liquids. Liquid vibrate, move about, and slide past each other. A gas is a state of matter lacking either a defined volume or defined shape. A supercritical fluid (scf) is a gas whose temperature and pressure are above the critical temperature and critical pressure respectively. Web the atoms and molecules in gases are much more spread out than in solids or liquids. Direct the children of grade 2 and grade 3 to observe the illustrations given in this circle. In the video here, sal uses a horizontal line through the phase diagram. No expand when heated expand greatly when heated. They can be crystalline, like table salt, or amorphous, like glass, rubber or plastic. As such, they can both be weighed, and have a fixed volume. Expand a little when heated. Highly strong intermolecular forces between the molecules, leads to a definite volume in solids.Solids, Liquids, & Gases! Rachel A Tall Drink of Water
Solids, Liquids, Gases Wall Chart Rapid Online
States of Matter Anchor Chart Classroom Decor Posters for Solid Liquid
Solids Liquids And Gases Poster
Learning about matter and its various states. Anchor charts, Solid
States of Matter NurseHub
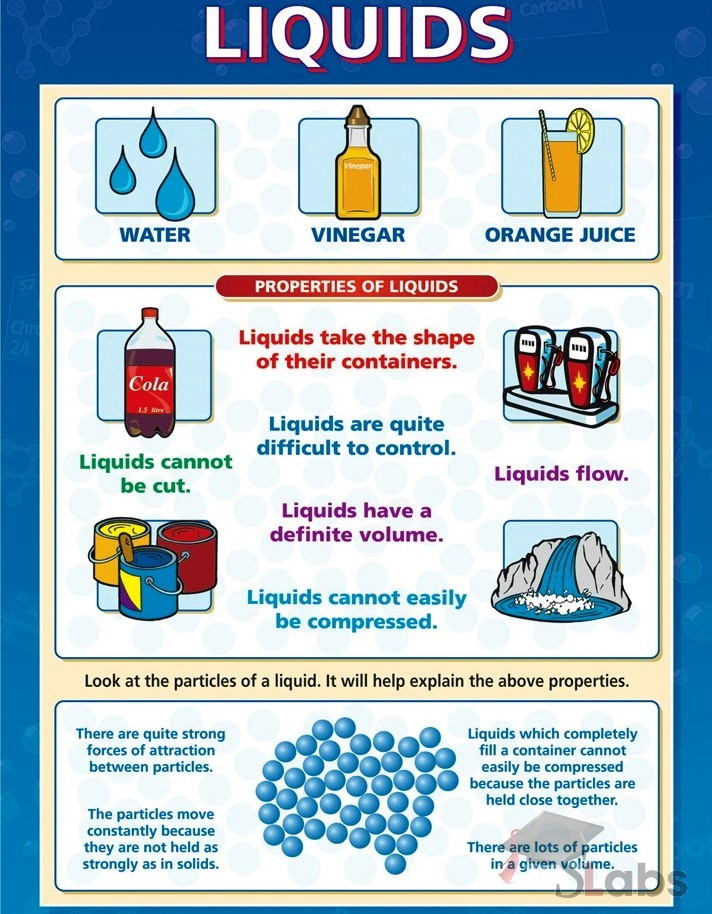
Solids, Liquids, Gases Chart Scholars Labs Chemistry classroom
What are states of matter? TheSchoolRun
States of Matter
Solids, Liquids, Gases Chart Scholars Labs
However, Water Isn’t The Only Type Of Matter That.
Gas Refers To A State Of Matter, Do Not Have Any Shape But Conform To The Shape Of The Container, Completely, In Which It Is Put In.
Web Solid Refers To A Form Of Matter Which Has Structural Rigidity And Has A Firm Shape Which Cannot Be Changed Easily.
A Gas Will Fill Any Container, But If The Container Is Not Sealed, The Gas Will Escape.
Related Post: