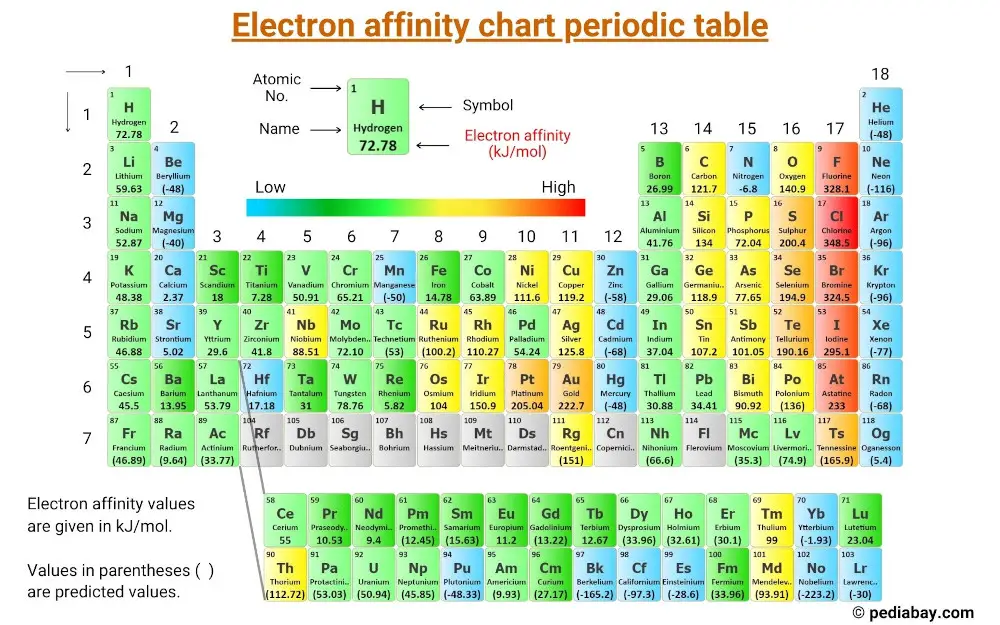
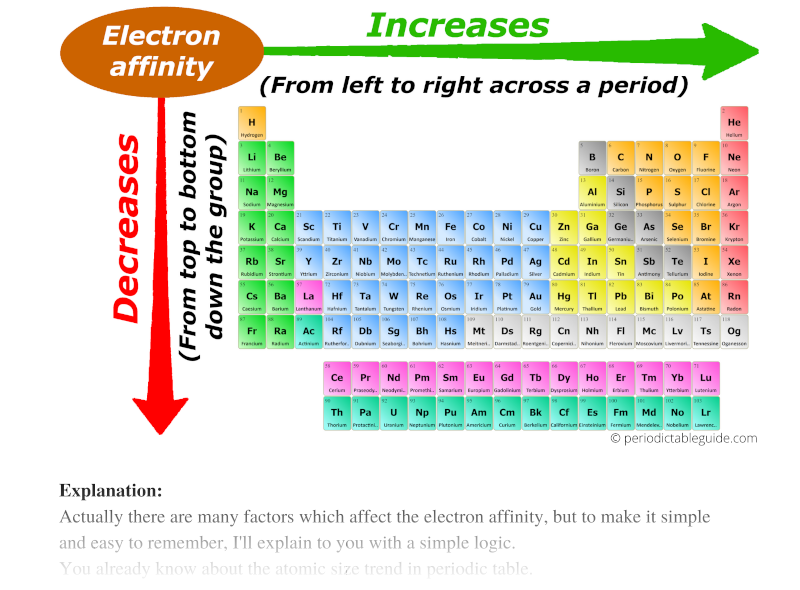
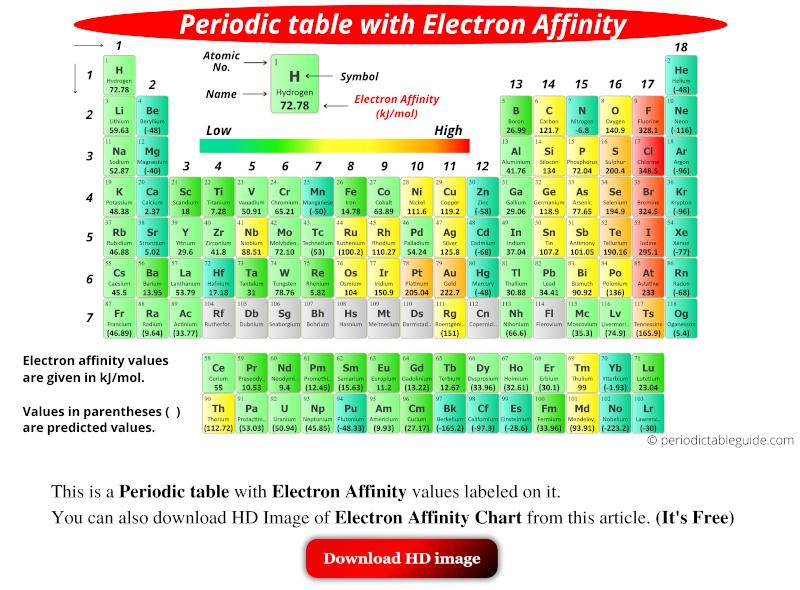
Chart Of Electron Affinity
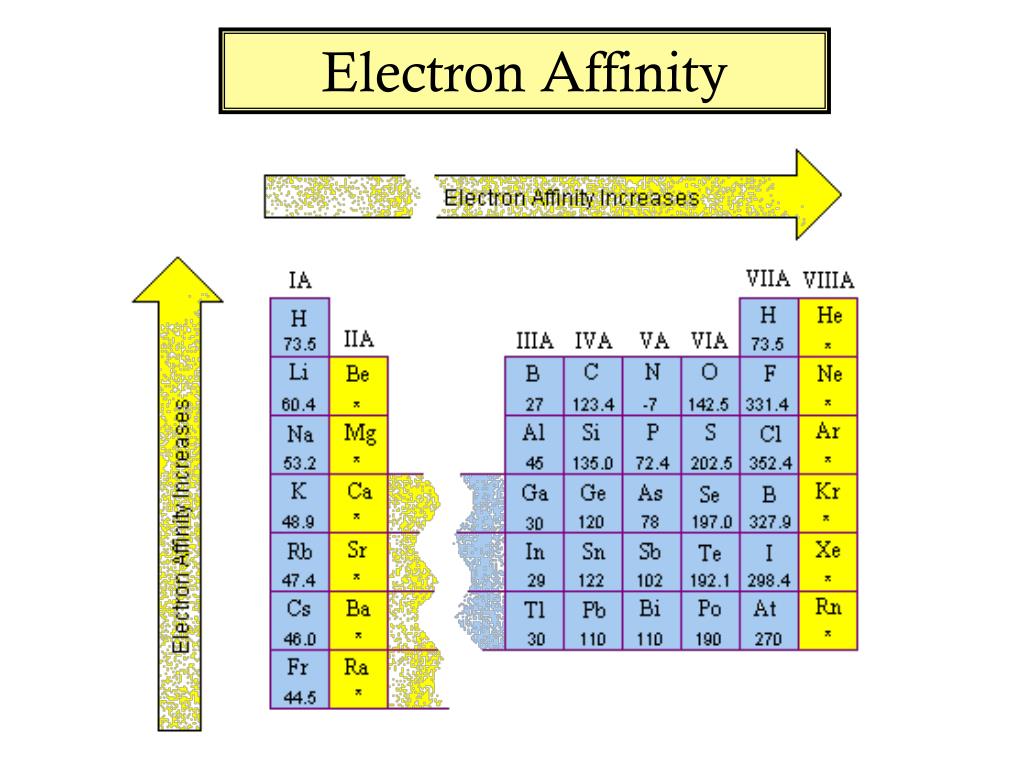
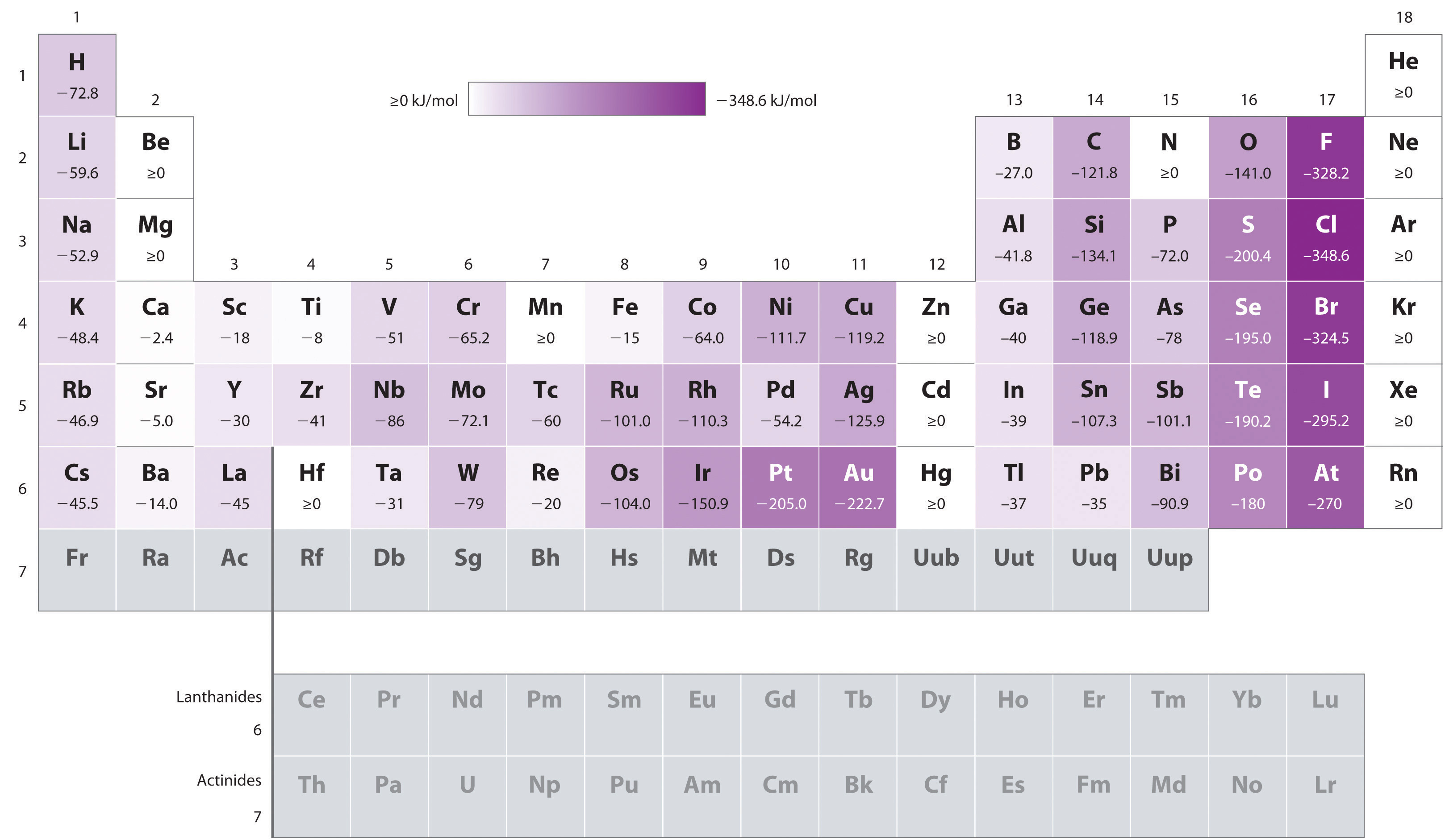
Chart Of Electron Affinity - In other words, the neutral atom's likelihood of gaining an electron. Web electron affinity is the energy change that results from adding an electron to a gaseous atom. \ [x_ { (g)} + e^− \rightarrow x^−_ { (g)} + energy\] z. The first electron affinities of the group 7 elements. Electron affinities decrease from top to bottom down groups. The figure below shows electron affinities in \(\ce{kj/mol}\) for the representative elements. Groups via and viia in the periodic table have the largest electron affinities. Electron affinity is the energy change when an atom gains an electron. Web periodic table with electron affinity. Web electron affinity chart for all the elements are given below. Web the electron affinity (e ea) of an atom or molecule is defined as the amount of energy release when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. Web the electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The figure below shows electron affinities in \(\ce{kj/mol}\) for the representative elements. These values are in kj/mol and the values written in parentheses ( ) are the predicted values. Web electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity also applies to molecules, in some cases. Web the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Web electron affinity for all the elements in the periodic table. The first electron affinities of the group 7 elements. You can also get the periodic table labeled with electron affinity values of elements. Web the most common units for electron affinity are kilojoules per mole (kj/mol) or electronvolts (ev). \ [x_ { (g)} + e^− \rightarrow x^−_ { (g)} + energy\] z. Electron affinity is the energy change when an atom gains an electron. The amount of energy released. The first electron affinities of the group 7 elements. These values are in kj/mol and the values written in parentheses ( ) are the predicted values. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. Electron. As discussed, electron affinities increase from left to right across periods; The first electron affinities of the group 7 elements. Web explore how electron affinity changes with atomic number in the periodic table of elements via interactive plots. So the more negative the electron affinity the more favourable the electron addition process is. Web the electron affinity is defined as. Web table shows electron affinity (i.e. First electron affinities have negative values. Electron affinity (ev) electron affinity (kj/mol) 1. Web electron affinity is the energy change that results from adding an electron to a gaseous atom. Web electron affinity chart for all the elements are given below. Web the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Web electron affinity is the energy change that results from adding an electron to a gaseous atom. Web the energy change that occurs when a neutral atom gains an electron is called its. Small numbers indicate that a less stable negative ion is formed. First electron affinities have negative values. For most elements, except noble gases, this is an exothermic process. Web electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative. These values are in kj/mol and the values written in parentheses ( ) are the predicted values. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The equivalent more common definition is the energy released (e initial+ e final) when an additional electron is attached to. In other words, the neutral atom's likelihood of gaining an electron. Electron affinities decrease from top to bottom down groups. Web periodic table with electron affinity. As discussed, electron affinities increase from left to right across periods; When energy is released in a chemical reaction or process, that energy is expressed as a negative number. These values are in kj/mol and the values written in parentheses ( ) are the predicted values. First, as the energy that is released by adding an electron to an isolated gaseous atom. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and. By convention, the negative sign shows a release of energy. X(g) + e − → x − (g) + energy Groups via and viia in the periodic table have the largest electron affinities. Small numbers indicate that a less stable negative ion is formed. You can also get the periodic table labeled with electron affinity values of elements. Web electron affinity can be defined in two equivalent ways. Web electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion. Web the most common units for electron affinity are kilojoules per mole (kj/mol) or electronvolts (ev). For most elements, except noble gases, this is an exothermic process. You can also get the periodic table labeled with electron affinity values of elements. How to find electron affinity Electron affinity also applies to molecules, in some cases. Electron affinities decrease from top to bottom down groups. By convention, the negative sign shows a release of energy. Web the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. As discussed, electron affinities increase from left to right across periods; Electron affinity (ev) electron affinity (kj/mol) 1. So the more negative the electron affinity the more favourable the electron addition process is. Web electron affinity chart for all the elements are given below. The amount of energy released when an electron is added to atom) for most of chemical elements. Small numbers indicate that a less stable negative ion is formed.Electron Affinity Trend and Definition
Electron Affinity Chemistry Steps
PPT Chapter 4 The Periodic Table PowerPoint Presentation, free
1.1.2.4 Electron Affinity Chemistry LibreTexts
Periodic Behavior Presentation Chemistry
Electron Affinity Chart of Elements (With Periodic Table) Pediabay
How about electron affinity? Electron affinity, Ionization energy
All Periodic Trends in Periodic Table (Explained with Image)
Electron Affinity Definition, Chart & Trend in Periodic Table
Electron Affinity Chart (Labeled Periodic table + List)
In General, Elements With The Most Negative Electron Affinities (The Highest Affinity For An Added Electron) Are Those With The Smallest Size And Highest Ionization Energies And Are Located In The Upper Right.
Web Table Shows Electron Affinity (I.e.
Web The Electron Affinity (E Ea) Of An Atom Or Molecule Is Defined As The Amount Of Energy Release When An Electron Attaches To A Neutral Atom Or Molecule In The Gaseous State To Form An Anion.
Web Electron Affinity For All The Elements In The Periodic Table.
Related Post:

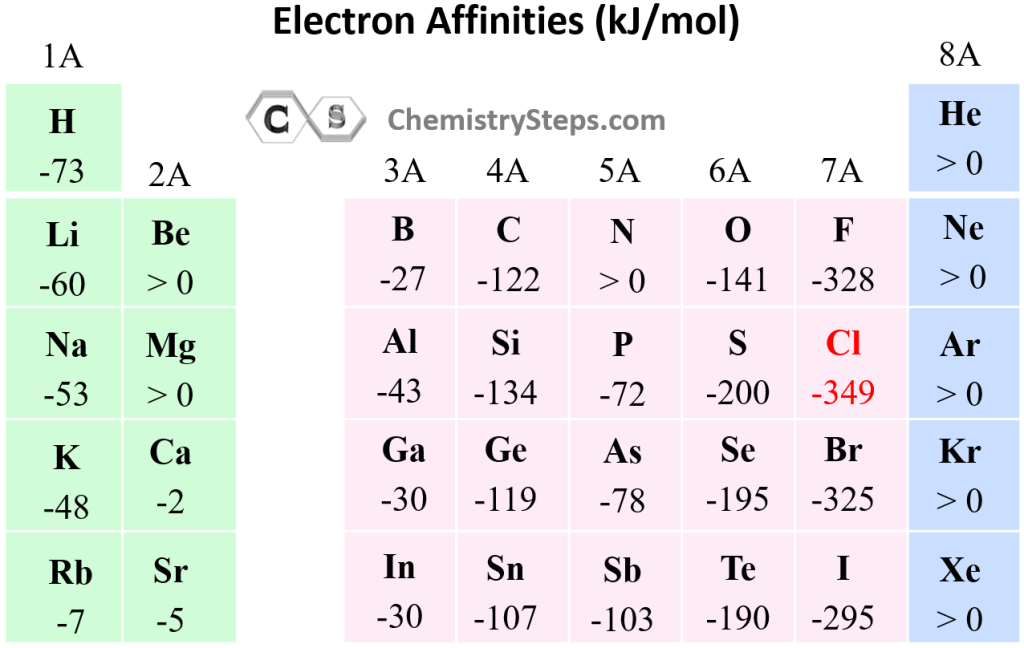
.PNG)